Prinsip Aufbau: Perbedaan antara revisi
Tidak ada ringkasan suntingan |
k Menambahkan kutipan |
||
| (14 revisi perantara oleh 8 pengguna tidak ditampilkan) | |||
| Baris 1: | Baris 1: | ||
[[Berkas:Klechkovski rule.svg|jmpl|upright=1.5|Urutan di mana orbital diatur berdasarkan kenaikan energi sesuai dengan aturan Madelung. Setiap panah merah diagonal menyatakan nilai yang berbeda dari {{nowrap|''n + ℓ''.}}]] |
|||
<!--{{redirect|Atomic build-up|the spread of nuclear weapons|Nuclear proliferation}} |
|||
The '''Aufbau principle''' states that, hypothetically, [[electron]]s orbiting one or more [[atom]]s fill the lowest available [[energy level]]s before filling higher levels (e.g., 1s before 2s). In this way, the electrons of an [[atom]], [[molecule]], or [[ion]] harmonize into the most stable [[electron configuration]] possible. |
|||
'''Prinsip Aufbau''' menyatakan bahwa, secara hipotetis, [[elektron]] yang mengorbit satu atau lebih [[atom]] mengisi [[tingkat energi]] terendah yang tersedia sebelum mengisi tingkat yang lebih tinggi (misalnya, 1s sebelum 2s). Dengan cara ini, elektron pada [[atom]], [[molekul]], atau [[ion]] menyelaraskan ke [[konfigurasi elektron]] yang paling stabil. |
|||
''[[wikt:Aufbau|Aufbau]]'' is a German noun that means "construction". The Aufbau principle is sometimes called the '''building-up principle''' or the '''Aufbau rule'''. |
|||
| ⚫ | ''[[wikt:Aufbau|Aufbau]]'' adalah kata benda bahasa Jerman yang berarti "konstruksi". Prinsip Aufbau kadang-kadang disebut '''prinsip membangun''' atau '''aturan Aufbau'''. Menurut prinsip Aufbau ini elektron di dalam suatu atom akan berada dalam kondisi yang stabil bila mempunyai energi yang rendah, sedangkan elektron-elektron akan berada pada orbital-orbital yang bergabung membentuk subkulit.<ref name=":0">{{cite|last=Chang|first=R.|year=2007|title=Kimia Dasar (terj)|publisher=Erlangga|location=Jakarta}}</ref> Jadi, elektron mempunyai kecenderungan akan menempati subkulit yang tingkat energinya rendah.<ref name=":1">{{cite|last=Keenan|year=1990|title=General Chemistry|publisher=Elsiever|location=Boston}}</ref> |
||
The details of this "building-up" tendency are described mathematically by [[atomic orbital]] functions. Electron behavior is elaborated by other principles of [[atomic physics]], such as [[Hund's rule]] and the [[Pauli exclusion principle]]. Hund's rule asserts that even if [[Degenerate orbitals|multiple orbitals of the same energy]] are available, electrons fill unoccupied orbitals first, before reusing orbitals occupied by other electrons. But, according to the Pauli exclusion principle, in order for electrons to occupy the same orbital, they must have different [[Spin (physics)|spin]]s (-1/2 and 1/2). |
|||
Rincian kecenderungan "membangun" ini dijelaskan secara matematis bedasarkan fungsi [[orbital atom]]. Perilaku elektron diuraikan oleh prinsip lain [[fisika atom]], seperti [[aturan Hund]] dan [[asas larangan Pauli]]. Aturan Hund menegaskan bahwa bahkan jika [[Orbital terdegenerasi|beberapa orbital]] dari energi yang sama yang tersedia, elektron mengisi orbital kosong pertama, sebelum menggunakan kembali orbital yang ditempati oleh elektron lainnya. Hal tersebut terjadi karena pada kenyataannya semua elektron memiliki muatan listrik yang sama, sehingga ada kecendrungan bagi elektron-elektron tersebut untuk mencari orbital kosong dengan energi sama sebelum berpasangan dengan elektron yang telah terlebih dahulu mengisi setengah orbital<ref>{{Cite book|last=Cahyana|first=Ucu|date=2007|url=http://repository.ut.ac.id/4587/2/PEKI4204-M1.pdf|title=Kimia Anorganik 1|location=Tangerang Selatan|publisher=Universitas Terbuka|isbn=9790110235|pages=1.34|url-status=live}}</ref>. Tetapi berdasarkan prinsip pengecualian Pauli, syarat agar elektron dapat mengisi orbital yang sama, mereka harus mempunyai [[putaran elektron]] yang berbeda (-1/2 dan 1/2). |
|||
| ⚫ | |||
| ⚫ | Satu versi prinsip Aufbau dikenal sebagai [[model kulit nuklir]] digunakan untuk memperkirakan konfigurasi [[proton]] dan [[neutron]] dalam [[inti atom]].<ref>{{cite book|last1 = Cottingham|first1 = W. N.|last2 = Greenwood|first2 = D. A.|title = An introduction to nuclear physics|publisher = Cambridge University Press|date = 1986|ISBN = 0 521 31960 9|chapter = Chapter 5: Ground state properties of nuclei: the shell model }}</ref> |
||
==Madelung energy ordering rule== |
|||
<!-- |
|||
[[File:Klechkovski rule.svg|thumb|upright=1.5|Order in which orbitals are arranged by increasing energy according to the Madelung rule. Each diagonal red arrow corresponds to a different value of {{nowrap|''n + ℓ''.}}]] |
|||
== Aturan pengurutan energi Madelung == |
|||
The order in which these orbitals are filled is given by the ''n + |
The order in which these orbitals are filled is given by the ''n + ℓ rule'', also known as the '''Madelung rule''' (after [[Erwin Madelung]]), or the '''Janet rule''' or the '''Klechkowski rule''' (after [[Charles Janet]] or [[Vsevolod Klechkovsky]] in some, mostly French and Russian-speaking, countries), or the '''diagonal rule'''.<ref>{{cite web | url = http://www.wyzant.com/resources/lessons/science/chemistry/electron_configuration | title = Electron Configuration | publisher = [[WyzAnt]] }}</ref> Orbitals with a lower ''n + ℓ'' value are filled before those with higher ''n + ℓ'' values. In this context, ''n'' represents the [[principal quantum number]] and ''ℓ'' the [[azimuthal quantum number]]; the values ''ℓ'' = 0, 1, 2, 3 correspond to the ''s'', ''p'', ''d'', and ''f'' labels, respectively. |
||
The rule is based on the total number of nodes in the atomic orbital, ''n + |
The rule is based on the total number of nodes in the atomic orbital, ''n + ℓ'', which is related to the energy.<ref>{{cite book |last1 = Weinhold | first1 = Frank | last2 = Landis | first2 = Clark R. |title=Valency and bonding: A Natural Bond Orbital Donor-Acceptor Perspective |location=Cambridge |publisher=Cambridge University Press | date = 2005 | pages = 715–716 |isbn=0-521-83128-8}}</ref> In the case of equal ''n + ℓ'' values, the orbital with a lower ''n'' value is filled first. The fact that most of the ground state configurations of neutral atoms fill orbitals following this ''n + ℓ, n'' pattern was obtained experimentally, by reference to the spectroscopic characteristics of the elements.<ref>{{cite journal |last=Scerri |first=Eric R. |title=How Good is the Quantum Mechanical Explanation of the Periodic System? |journal=[[Journal of Chemical Education|J. Chem. Ed.]] |volume=75 |issue=11 |pages=1384–85 |date=1998 |url= http://www.chem.ucla.edu/dept/Faculty/scerri/pdf/How_Good_is.pdf |doi=10.1021/ed075p1384 |bibcode=1998JChEd..75.1384S }}</ref> |
||
The Madelung energy ordering rule applies only to neutral atoms in their ground state, and even in that case, there are several elements for which it predicts configurations that differ from those determined experimentally.<ref>{{cite journal |last=Meek |first=Terry L. | last2 = Allen | first2 = Leland C. |title=Configuration irregularities: deviations from the Madelung rule and inversion of orbital energy levels |journal=[[Chemical Physics Letters|Chem. Phys. Lett.]] |volume=362 |issue=5–6 |pages=362–64 |doi=10.1016/S0009-2614(02)00919-3 |date=2002 |bibcode=2002CPL...362..362M }}</ref> [[Copper]], [[chromium]], and [[palladium]] are common examples of this property. According to the Madelung rule, the 4s orbital (''n + |
The Madelung energy ordering rule applies only to neutral atoms in their ground state, and even in that case, there are several elements for which it predicts configurations that differ from those determined experimentally.<ref>{{cite journal |last=Meek |first=Terry L. | last2 = Allen | first2 = Leland C. |title=Configuration irregularities: deviations from the Madelung rule and inversion of orbital energy levels |journal=[[Chemical Physics Letters|Chem. Phys. Lett.]] |volume=362 |issue=5–6 |pages=362–64 |doi=10.1016/S0009-2614(02)00919-3 |date=2002 |bibcode=2002CPL...362..362M }}</ref> [[Copper]], [[chromium]], and [[palladium]] are common examples of this property. According to the Madelung rule, the 4s orbital (''n + ℓ'' = 4 + 0 = 4) is occupied before the 3d orbital (''n + ℓ'' = 3 + 2 = 5). The rule then predicts the configuration of <sub>29</sub>Cu to be 1s<sup>2</sup>2s<sup>2</sup>2p<sup>6</sup>3s<sup>2</sup> 3p<sup>6</sup>4s<sup>2</sup>3d<sup>9</sup>, abbreviated [Ar]4s<sup>2</sup>3d<sup>9</sup> where [Ar] denotes the configuration of Ar (the preceding noble gas). However the experimental electronic configuration of the copper atom is [Ar]4s<sup>1</sup>3d<sup>10</sup>. By filling the 3d orbital, copper can be in a lower energy state. Similarly, chromium takes the electronic configuration of [Ar]4s<sup>1</sup>3d<sup>5</sup> instead of [Ar]4s<sup>2</sup>3d<sup>4</sup>. In this case, chromium has a half-full 3d shell. For palladium, the Madelung rule predicts [Kr]5s<sup>2</sup>4d<sup>8</sup>, but the experimental configuration [Kr]4d<sup>10</sup> differs in the placement of two electrons. |
||
| ⚫ | |||
== Sejarah == |
|||
=== Prinsip Aufbau dalam teori kuantum baru === |
|||
==History== |
|||
[[Berkas:Sommerfeld ellipses.svg|jmpl|Dalam [[teori kuantum lama]], orbit dengan momentum sudut rendah (''s''- dan ''p''- orbital) lebih dekat ke inti.]] |
|||
Prinsip ini mengambil namanya dari Jerman, ''Aufbauprinzip'', "prinsip membangun", bukannya diberi nama seorang ilmuwan. Bahkan, ia dirumuskan oleh [[Niels Bohr]] dan [[Wolfgang Pauli]] di awal 1920-an, dan menyatakan bahwa: |
|||
{{cquote|Orbital energi yang lebih rendah diisi pertama dengan elektron dan hanya kemudian orbital energi tinggi dipenuhi.}} |
|||
===The Aufbau principle in the new quantum theory=== |
|||
[[File:Sommerfeld ellipses.svg|thumb|In the [[old quantum theory]], orbits with low angular momentum (''s''- and ''p''-orbitals) get closer to the nucleus.]] |
|||
The principle takes its name from the German, ''Aufbauprinzip'', "building-up principle", rather than being named for a scientist. In fact, it was formulated by [[Niels Bohr]] and [[Wolfgang Pauli]] in the early 1920s, and states that: |
|||
Ini merupakan sebuah aplikasi awal [[mekanika kuantum]] untuk sifat-sifat [[elektron]], dan menjelaskan sifat kimia dalam hal fisik. Setiap elektron ditambahkan tunduk pada medan listrik dibuat oleh muatan positif dari [[inti atom]] ''dan'' muatan negatif elektron lainnya yang terikat untuk inti. Meskipun dalam hidrogen tidak ada perbedaan energi antara orbital dengan bilangan kuantum utama yang sama ''n'', hal ini tidak berlaku untuk elektron terluar dari atom lain. |
|||
{{cquote|The orbitals of lower energy are filled in first with the electrons and only then the orbitals of high energy are filled.}} |
|||
Dalam [[teori kuantum lama]] sebelum [[mekanika kuantum]], elektron seharusnya menempati orbit elips klasik. Orbit dengan momentum sudut tertinggi adalah 'orbit lingkaran' di luar elektron dalam, tapi orbit dengan momentum sudut rendah (''s''- dan ''p''- orbital) memiliki eksentrisitas orbit yang tinggi, sehingga mereka lebih dekat dengan inti dan merasa rata-rata muatan nuklir kurang kuat disaring. |
|||
This was an early application of [[quantum mechanics]] to the properties of [[electron]]s, and explained chemical properties in [[physics|physical]] terms. Each added electron is subject to the electric field created by the positive charge of the [[atomic nucleus]] ''and'' the negative charge of other electrons that are bound to the nucleus. Although in hydrogen there is no energy difference between orbitals with the same principal quantum number ''n'', this is not true for the outer electrons of other atoms. |
|||
<!-- |
|||
=== Aturan pengurutan energi ''n + ℓ'' === |
|||
| ⚫ | A periodic table in which each row corresponds to one value of ''n + ℓ'' was suggested by [[Charles Janet]] in 1927. In 1936, the German physicist [[Erwin Madelung]] proposed his empirical rules for the order of filling atomic subshells, based on knowledge of atomic ground states determined by the analysis of atomic spectra, and most English-language sources therefore refer to the Madelung rule. Madelung may have been aware of this pattern as early as 1926.<ref>{{cite journal |title= The Order of Electron Shells in Ionized Atoms |last1= Goudsmit |first1= S. A. |last2= Richards |first2= Paul I. |journal= [[Proceedings of the National Academy of Sciences of the United States of America|Proc. Natl. Acad. Sci.]] |pages= 664–671 (with correction on p 906) |volume= 51 | issue= 4 |date= 1964 |url= http://www.pnas.org/content/51/4/664.full.pdf |bibcode = 1964PNAS...51..664G |doi = 10.1073/pnas.51.4.664 }}</ref> In 1962 the Russian agricultural chemist [[V.M. Klechkovsky|V.M. Klechkowski]] proposed the first theoretical explanation for the importance of the sum ''n + ℓ'', based on the statistical [[Thomas–Fermi model]] of the atom.<ref>{{cite journal | title = Theoretical justification of Madelung's rule | journal = [[Journal of Chemical Education|J. Chem. Ed.]] | url = http://jchemed.chem.wisc.edu/Journal/Issues/1979/Nov/jceSubscriber/JCE1979p0714.pdf | last = Wong | first = D. Pan | date = 1979 | issue = 11 | pages = 714–718 | volume = 56 | doi = 10.1021/ed056p714|bibcode = 1979JChEd..56..714W }}</ref> Many French- and Russian-language sources therefore refer to the Klechkowski rule. |
||
Dalam beberapa tahun terakhir beberapa penulis telah menantang keabsahan aturan Madelung dalam memprediksi urutan pengisian orbital atom. Sebagai contoh, telah diklaim, bukan untuk pertama kalinya, bahwa dalam kasus atom skandium orbital 3d ditempati 'sebelum' pendudukan orbital 4s. Selain sana menjadi banyak bukti eksperimental untuk mendukung pandangan ini, itu membuat penjelasan dari urutan ionisasi elektron dalam ini dan logam transisi lainnya jauh lebih dimengerti, mengingat bahwa 4s elektron selalu secara istimewa terionisasi.<ref>{{cite journal | title = The Trouble With the Aufbau Principle | journal = [[Education in Chemistry]] | url = http://www.rsc.org/eic/2013/11/aufbau-electron-configuration | last = Scerri | first = Eric | date = 2013 | issue = 11 | pages = 24–26 | volume = 50 }}</ref> |
|||
--> |
|||
In the [[old quantum theory]] prior to [[quantum mechanics]], electrons were supposed to occupy classical elliptical orbits. The orbits with the highest angular momentum are 'circular orbits' outside the inner electrons, but orbits with low angular momentum (''s''- and ''p''-orbitals) have high [[orbital eccentricity]], so that they get closer to the nucleus and feel on average a less strongly screened nuclear charge. |
|||
== Urutan konfigurasi == |
|||
===The ''n + ℓ'' energy ordering rule=== |
|||
| ⚫ | |||
| ⚫ | A periodic table in which each row corresponds to one value of ''n + |
||
In recent years some authors have challenged the validity of Madelung's rule in predicting the order of filling of atomic orbitals. For example, it has been claimed, not for the first time, that in the case of the scandium atom a 3d orbital is occupied 'before' the occupation of the 4s orbital. In addition to there being ample experimental evidence to support this view, it makes the explanation of the order of ionization of electrons in this and other transition metals far more intelligible, given that 4s electrons are invariably preferentially ionized.<ref>{{cite journal | title = The Trouble With the Aufbau Principle | journal = [[Education in Chemistry]] | url = http://www.rsc.org/eic/2013/11/aufbau-electron-configuration | last = Scerri | first = Eric | date = 2013 | issue = 11 | pages = 24–26 | volume = 50 }}</ref> |
|||
| ⚫ | Urutan-urutan tingkat energi di tujukan pada gambar di samping kanan. Jadi pengisian orbital dimulai dari orbital 1s, 2s, 2p, dan seterusnya. Pada gambar dapat dilihat bahwa subkulit 3d mempunyai energi lebih tinggi daripada subkulit 4s.<ref name=":0">{{cite|last=Chang|first=R.|year=2007|title=Kimia Dasar (terj)|publisher=Erlangga|location=Jakarta}}</ref> Oleh karena itu, setelah 3p terisi penuh maka elektron berikutnya akan mengisi subkulit 4s, baru kemudian akan mengisi sub kulit 3d.<ref name=":1">{{cite|last=Keenan|year=1990|title=General Chemistry|publisher=Elsiever|location=Boston}}</ref> |
||
==See also== |
|||
* [[Electron configuration]] |
|||
* [[Valence electrons]] |
|||
* [[Wiswesser's rule]] |
|||
| ⚫ | |||
==References== |
|||
| ⚫ | |||
<references/> |
|||
| ⚫ | |||
| ⚫ | |||
== |
== Lihat pula == |
||
* [[Konfigurasi elektron]] |
|||
| ⚫ | |||
* [[Elektron valensi]] |
|||
| ⚫ | |||
* [[Teori atom]] |
|||
| ⚫ | *{{cite journal | |
||
| ⚫ | *{{cite journal | |
||
| ⚫ | |||
| ⚫ | *{{cite journal | |
||
== |
== Referensi == |
||
{{Reflist|30em}} |
|||
| ⚫ | |||
== Bacaan lebih lanjut == |
|||
[[Category:Electron states]] |
|||
| ⚫ | |||
[[Category:Foundational quantum physics]] |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
== Pengertian == |
|||
| ⚫ | * {{cite journal |title=On the dynamical symmetry of the periodic table. II. Modified Demkov-Ostrovsky atomic model |first1=Y. |last1=Kitagawara |year=1984 |journal=J. Phys. B |volume=17 |issue=21 |pages=4251–59 |doi=10.1088/0022-3700/17/21/013|last2=Barut |first2=A.O. |bibcode=1984JPhB...17.4251K }} |
||
| ⚫ | Aufbau berarti membangun. Menurut prinsip Aufbau ini elektron di dalam suatu atom akan berada dalam kondisi yang stabil bila mempunyai energi yang rendah, sedangkan elektron-elektron akan berada pada orbital-orbital yang bergabung membentuk subkulit<ref name=":0">{{cite|last=Chang|first=R.|year=2007|title=Kimia Dasar (terj)|publisher=Erlangga|location=Jakarta}}</ref> |
||
| ⚫ | |||
| ⚫ | * {{cite journal |title=Transition Metals and the Aufbau Principle |first=L. G. |last=Vanquickenborne |year=1994 |journal=Journal of Chemical Education |volume=71 |issue=6 |pages=469–471 |url=http://depa.fquim.unam.mx/amyd/archivero/4svs3d_26264.pdf |bibcode=1994JChEd..71..469V |doi=10.1021/ed071p469 }} |
||
== |
== Pranala luar == |
||
| ⚫ | |||
| ⚫ | Urutan-urutan tingkat energi di tujukan pada gambar di samping kanan. Jadi pengisian orbital dimulai dari orbital 1s, 2s, 2p, dan seterusnya. Pada gambar dapat dilihat bahwa subkulit 3d mempunyai energi lebih tinggi daripada subkulit 4s<ref name=":0" /> |
||
| ⚫ | |||
</gallery> |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
== Referensi == |
|||
{{reflist}} |
|||
[[Kategori: |
[[Kategori:Elektron]] |
||
[[Kategori:Kimia kuantum]] |
|||
[[Kategori:Fisika kuantum]] |
|||
Revisi terkini sejak 12 Desember 2023 04.39

Prinsip Aufbau menyatakan bahwa, secara hipotetis, elektron yang mengorbit satu atau lebih atom mengisi tingkat energi terendah yang tersedia sebelum mengisi tingkat yang lebih tinggi (misalnya, 1s sebelum 2s). Dengan cara ini, elektron pada atom, molekul, atau ion menyelaraskan ke konfigurasi elektron yang paling stabil.
Aufbau adalah kata benda bahasa Jerman yang berarti "konstruksi". Prinsip Aufbau kadang-kadang disebut prinsip membangun atau aturan Aufbau. Menurut prinsip Aufbau ini elektron di dalam suatu atom akan berada dalam kondisi yang stabil bila mempunyai energi yang rendah, sedangkan elektron-elektron akan berada pada orbital-orbital yang bergabung membentuk subkulit.[1] Jadi, elektron mempunyai kecenderungan akan menempati subkulit yang tingkat energinya rendah.[2]
Rincian kecenderungan "membangun" ini dijelaskan secara matematis bedasarkan fungsi orbital atom. Perilaku elektron diuraikan oleh prinsip lain fisika atom, seperti aturan Hund dan asas larangan Pauli. Aturan Hund menegaskan bahwa bahkan jika beberapa orbital dari energi yang sama yang tersedia, elektron mengisi orbital kosong pertama, sebelum menggunakan kembali orbital yang ditempati oleh elektron lainnya. Hal tersebut terjadi karena pada kenyataannya semua elektron memiliki muatan listrik yang sama, sehingga ada kecendrungan bagi elektron-elektron tersebut untuk mencari orbital kosong dengan energi sama sebelum berpasangan dengan elektron yang telah terlebih dahulu mengisi setengah orbital[3]. Tetapi berdasarkan prinsip pengecualian Pauli, syarat agar elektron dapat mengisi orbital yang sama, mereka harus mempunyai putaran elektron yang berbeda (-1/2 dan 1/2).
Satu versi prinsip Aufbau dikenal sebagai model kulit nuklir digunakan untuk memperkirakan konfigurasi proton dan neutron dalam inti atom.[4]
Sejarah
[sunting | sunting sumber]Prinsip Aufbau dalam teori kuantum baru
[sunting | sunting sumber]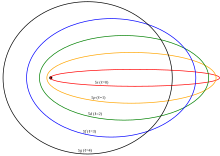
Prinsip ini mengambil namanya dari Jerman, Aufbauprinzip, "prinsip membangun", bukannya diberi nama seorang ilmuwan. Bahkan, ia dirumuskan oleh Niels Bohr dan Wolfgang Pauli di awal 1920-an, dan menyatakan bahwa:
Orbital energi yang lebih rendah diisi pertama dengan elektron dan hanya kemudian orbital energi tinggi dipenuhi.
Ini merupakan sebuah aplikasi awal mekanika kuantum untuk sifat-sifat elektron, dan menjelaskan sifat kimia dalam hal fisik. Setiap elektron ditambahkan tunduk pada medan listrik dibuat oleh muatan positif dari inti atom dan muatan negatif elektron lainnya yang terikat untuk inti. Meskipun dalam hidrogen tidak ada perbedaan energi antara orbital dengan bilangan kuantum utama yang sama n, hal ini tidak berlaku untuk elektron terluar dari atom lain.
Dalam teori kuantum lama sebelum mekanika kuantum, elektron seharusnya menempati orbit elips klasik. Orbit dengan momentum sudut tertinggi adalah 'orbit lingkaran' di luar elektron dalam, tapi orbit dengan momentum sudut rendah (s- dan p- orbital) memiliki eksentrisitas orbit yang tinggi, sehingga mereka lebih dekat dengan inti dan merasa rata-rata muatan nuklir kurang kuat disaring.
Urutan konfigurasi
[sunting | sunting sumber]Urutan-urutan tingkat energi di tujukan pada gambar di samping kanan. Jadi pengisian orbital dimulai dari orbital 1s, 2s, 2p, dan seterusnya. Pada gambar dapat dilihat bahwa subkulit 3d mempunyai energi lebih tinggi daripada subkulit 4s.[1] Oleh karena itu, setelah 3p terisi penuh maka elektron berikutnya akan mengisi subkulit 4s, baru kemudian akan mengisi sub kulit 3d.[2]
Langkah-langkah penulisan konfigurasi elektron
[sunting | sunting sumber]- Menentukan jumlah elektron dari atom tersebut. Jumlah elektron dari atom unsur sama dengan nomor atom unsur tersebut.
- Menuliskan jenis subkulit yang dibutuhkan secara urut berdasarkan diagram curah hujan pada gambar 2 yaitu: 1s- 2s- 2p- 3s- 3p- 4s- 3d- 4p- 5s- 4d- 5p- 6s- 4f- 5d- 6p- 7s- 5f- 6d- 7p- 8s
- Mengisikan elektron pada masing-masing subkulit dengan memperhatikan jumlah elektron maksimumnya, maka sisa elektron dimasukan pada subkulit berikutnya.[2]
Lihat pula
[sunting | sunting sumber]Referensi
[sunting | sunting sumber]- ^ a b Chang, R. (2007), Kimia Dasar (terj), Jakarta: Erlangga
- ^ a b c Keenan (1990), General Chemistry, Boston: Elsiever
- ^ Cahyana, Ucu (2007). Kimia Anorganik 1 (PDF). Tangerang Selatan: Universitas Terbuka. hlm. 1.34. ISBN 9790110235.
- ^ Cottingham, W. N.; Greenwood, D. A. (1986). "Chapter 5: Ground state properties of nuclei: the shell model". An introduction to nuclear physics. Cambridge University Press. ISBN 0 521 31960 9.
Bacaan lebih lanjut
[sunting | sunting sumber]- Image: Understanding order of shell filling Diarsipkan 2014-11-15 di Wayback Machine.
- Boeyens, J. C. A.: Chemistry from First Principles. Berlin: Springer Science 2008, ISBN 978-1-4020-8546-8
- Ostrovsky, V.N. (2005). "On Recent Discussion Concerning Quantum Justification of the Periodic Table of the Elements". Foundations of Chemistry. 7 (3): 235–39. doi:10.1007/s10698-005-2141-y.
- Kitagawara, Y.; Barut, A.O. (1984). "On the dynamical symmetry of the periodic table. II. Modified Demkov-Ostrovsky atomic model". J. Phys. B. 17 (21): 4251–59. Bibcode:1984JPhB...17.4251K. doi:10.1088/0022-3700/17/21/013.
- Scerri, E.R. (2013). "The Trouble with the Aufbau Principle". Education in Chemistry: 24–26.
- Vanquickenborne, L. G. (1994). "Transition Metals and the Aufbau Principle" (PDF). Journal of Chemical Education. 71 (6): 469–471. Bibcode:1994JChEd..71..469V. doi:10.1021/ed071p469.
