Asam laktat: Perbedaan antara revisi
Tampilan
Konten dihapus Konten ditambahkan
Reverted to revision 6231383 by EmausBot: nonsense. (TW) |
Tidak ada ringkasan suntingan Tag: Suntingan perangkat seluler Suntingan peramban seluler |
||
| (14 revisi perantara oleh 9 pengguna tidak ditampilkan) | |||
| Baris 1: | Baris 1: | ||
{{chembox |
{{chembox |
||
| Verifiedfields = changed |
|||
| Watchedfields = changed |
|||
| verifiedrevid = 477002503 |
|||
| Name = Asam laktat |
| Name = Asam laktat |
||
| ImageFile = |
|||
| ImageFileL1 = Lactic-acid-skeletal.svg |
|||
| ImageFileL1 = 7 Milchsäure.svg |
|||
| ImageSizeL1 = 120px |
|||
| ImageSizeL1 = 110 |
|||
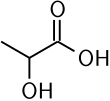
| ImageNameL1 = Skeletal formula of lactic acid |
|||
| ImageNameL1 = Skeletal formula of <small>L</small>-lactic acid |
|||
| ImageFileR1 = Lactic-acid-3D-balls.png |
|||
| |
| ImageCaptionL1 = |
||
| ImageFileR1 = File:L-Lactic acid molecule spacefill.png |
|||
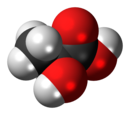
| ImageNameR1 = Ball-and-stick model of lactic acid |
|||
| ImageSizeR1 = 130 |
|||
| IUPACName = 2-hydroxypropanoic acid |
|||
| ImageNameR1 = |
|||
''Note:'' The S enantiomer is depicted in each of the structural models included above. |
|||
| ImageCaption2 = <small>rac</small>-Lactic acid |
|||
| PIN = 2-Hydroxypropanoic acid<ref name=iupac2013>{{cite book | title = Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book) | publisher = [[Royal Society of Chemistry|The Royal Society of Chemistry]] | date = 2014 | location = Cambridge | page = 748 | doi = 10.1039/9781849733069-00648 | isbn = 978-0-85404-182-4}}</ref> |
|||
| SystematicName = |
|||
| OtherNames = Lactic acid<ref name=iupac2013 /><br />Milk acid |
|||
| Section1 = {{Chembox Identifiers |
| Section1 = {{Chembox Identifiers |
||
| IUPHAR_ligand = 2932 |
|||
| CASNo= 50-21-5 |
|||
| CASNo_Ref = {{cascite|correct|CAS}} |
|||
| CASOther = <br /><small>D</small>/<small>L</small>: [50-21-5] <br /><small>L</small>: [79-33-4]<br /><small>D</small>: [10326-41-7] |
|||
| CASNo = 50-21-5 |
|||
| CASNo1_Ref = {{cascite|correct|CAS}} |
|||
| CASNo1 = 79-33-4 |
|||
| ChEMBL_Ref = {{ebicite|correct|EBI}} |
|||
| ChEMBL = 330546 |
|||
| CASNo1_Comment = (<small>L</small>) |
|||
| CASNo2_Ref = {{cascite|correct|CAS}} |
|||
| CASNo2 = 10326-41-7 |
|||
| CASNo2_Comment = (<small>D</small>) |
|||
| UNII_Ref = {{fdacite|changed|FDA}} |
|||
| UNII = 33X04XA5AT |
|||
| ChEBI_Ref = {{ebicite|correct|EBI}} |
|||
| ChEBI = 422 |
|||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|||
| StdInChI = 1S/C3H6O3/c1-2(4)3(5)6/h2,4H,1H3,(H,5,6)/t2-/m0/s1 |
|||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|||
| StdInChIKey = JVTAAEKCZFNVCJ-REOHCLBHSA-N |
|||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|||
| ChemSpiderID = 96860 |
| ChemSpiderID = 96860 |
||
| SMILES = CC(O)C(=O)O |
| SMILES = CC(O)C(=O)O |
||
}} |
|||
| Section2 = {{Chembox Properties |
|||
| C=3 | H=6 | O=3 |
|||
| MeltingPt =53 °C |
|||
| BoilingPtC = 122 |
|||
| BoilingPt_notes = @ 15 mmHg |
|||
| pKa = 3.86,<ref>{{cite book| vauthors = Dawson RM |displayauthors=etal|title=Data for Biochemical Research|location=Oxford|publisher=Clarendon Press|date=1959}}</ref> 15.1<ref>{{cite journal | vauthors = Silva AM, Kong X, Hider RC | title = Determination of the pKa value of the hydroxyl group in the alpha-hydroxycarboxylates citrate, malate and lactate by 13C NMR: implications for metal coordination in biological systems | journal = Biometals | volume = 22 | issue = 5 | pages = 771–8 | date = October 2009 | pmid = 19288211 | doi = 10.1007/s10534-009-9224-5 }}</ref> |
|||
}} |
|||
| Section3 = |
|||
| Section4 = {{Chembox Thermochemistry| DeltaHc = 1361.9 kJ/mol, 325.5 kcal/mol, 15.1 kJ/g, 3.61 kcal/g}} |
|||
| Section8 = {{Chembox Related |
|||
| OtherAnions = lactate |
|||
| OtherFunction_label = [[carboxylic acid]]s |
|||
| OtherFunction = [[acetic acid]]<br />[[glycolic acid]]<br />[[propionic acid]]<br />[[3-hydroxypropanoic acid]]<br />[[malonic acid]]<br />[[butyric acid]]<br />[[hydroxybutyric acid]] |
|||
| OtherCompounds = [[1-propanol]]<br />[[2-propanol]]<br />[[propionaldehyde]]<br />[[acrolein]]<br />[[sodium lactate]] |
|||
}} |
|||
| Section5 = |
|||
| Section6 = {{Chembox Pharmacology |
|||
| ATCCode_prefix = G01 |
| ATCCode_prefix = G01 |
||
| ATCCode_suffix = AD01 |
| ATCCode_suffix = AD01 |
||
| ATC_Supplemental = {{ATCvet|P53|AG02}} |
| ATC_Supplemental = {{ATCvet|P53|AG02}} |
||
}} |
|||
| Section7 = {{Chembox Hazards |
|||
| GHSPictograms = {{GHSp|GHS05}}<ref name="sigma">{{Sigma-Aldrich|sial|id=69785|name=DL-Lactic acid|access-date=20 July 2013}}</ref> |
|||
| HPhrases = {{H-phrases|315|318}}<ref name="sigma" /> |
|||
| PPhrases = {{P-phrases|280|305+351+338}}<ref name="sigma" /> |
|||
}} |
}} |
||
| |
| Section8 = {{Chembox Related |
||
| Formula = C<sub>3</sub>H<sub>6</sub>O<sub>3</sub> |
|||
| MolarMass = 90.08 g/mol |
|||
| MeltingPt =<small>L</small>: 53 °C<br /><small>D</small>: 53 °C<br /><small>D</small>/<small>L</small>: 16.8 °C |
|||
| BoilingPt = 122 °C @ 12 mmHg |
|||
| pKa = 3.86 at 25 °C |
|||
}} |
|||
| Section4 = {{Chembox Related |
|||
| OtherAnions = laktat |
| OtherAnions = laktat |
||
| Function = [[asam karboksilat]] |
| Function = [[asam karboksilat]] |
||
| Baris 33: | Baris 74: | ||
}} |
}} |
||
'''Asam |
'''Asam lakta''' atau '''asam susu''' (CH3-CHOH-COOH) adalah [[senyawa kimia]] penting dalam beberapa proses [[biokimia]]. Seorang ahli kimia [[Swedia]], [[Carl Wilhelm Scheele]], kali meng[[isolasi]]nya pada tahun [[1780]]. Secara struktur, ia adalah [[asam karboksilat]] dengan satu [[gugus hidroksil]] yang menempel pada gugus [[karboksil]]. Dalam [[air]], ia terlarut dengan melepas [[proton]] (H<sup>+</sup>), membentuk [[ion]] laktat. Asam ini juga larut dalam [[alkohol]] dan bersifat menyerap air (higroskopik). |
||
Asam ini memiliki simetri cermin ([[kiralitas]]), dengan dua [[isomer]]: asam <small>L</small>-(+)-laktat atau asam (''S'')-laktat dan, cerminannya, iasam <small>D</small>-(-)-laktat atau asam (''R'')-laktat. Hanya isomer yang pertama (S) aktif secara [[biologi]] |
Asam ini memiliki simetri cermin ([[kiralitas]]), dengan dua [[isomer]]: asam <small>L</small>-(+)-laktat atau asam (''S'')-laktat dan, cerminannya, iasam <small>D</small>-(-)-laktat atau asam (''R'')-laktat. Hanya isomer yang pertama (S) aktif secara [[biologi]] |
||
== Pranala luar == |
|||
* [http://www.smithsonianmag.com/science-nature/10022381.html Corn Plastic to the Rescue] {{Webarchive|url=https://web.archive.org/web/20131121122758/http://www.smithsonianmag.com/science-nature/10022381.html |date=2013-11-21 }} |
|||
* [http://www.webmd.com/a-to-z-guides/lactic-acid Lactic Acid: Information and Resources] |
|||
* [https://www.nytimes.com/2006/05/16/health/nutrition/16run.html Lactic Acid Is Not Muscles' Foe, It's Fuel] |
|||
* {{cite web |first=Matt |last=Fitzgerald |name-list-format=vanc |date=January 26, 2010 |title=The Lactic Acid Myths |work=Competitor Running |url=http://running.competitor.com/2010/01/training/the-lactic-acid-myths_7938 |access-date=2018-09-10 |archive-date=2018-09-24 |archive-url=https://web.archive.org/web/20180924110049/https://running.competitor.com/2010/01/training/the-lactic-acid-myths_7938 |dead-url=yes }} |
|||
[[Kategori:Asam organik]] |
|||
{{Portal|Kimia}} |
{{Portal|Kimia}} |
||
{{Authority control}} |
|||
[[ |
[[Kategori:Asam organik]] |
||
[[be:Малочная кіслата]] |
|||
[[be-x-old:Малочная кісьля]] |
|||
[[bg:Млечна киселина]] |
|||
[[ca:Àcid làctic]] |
|||
[[cs:Kyselina mléčná]] |
|||
[[cy:Asid lactig]] |
|||
[[da:Mælkesyre]] |
|||
[[de:Milchsäure]] |
|||
[[el:2-υδροξυπροπανικό οξύ]] |
|||
[[en:Lactic acid]] |
|||
[[eo:Lakta acido]] |
|||
[[es:Ácido láctico]] |
|||
[[et:Piimhape]] |
|||
[[eu:Azido laktiko]] |
|||
[[fa:اسید لاکتیک]] |
|||
[[fi:Maitohappo]] |
|||
[[fr:Acide lactique]] |
|||
[[gl:Ácido láctico]] |
|||
[[he:חומצה לקטית]] |
|||
[[hr:Mliječna kiselina]] |
|||
[[hu:Tejsav]] |
|||
[[is:Mjólkursýra]] |
|||
[[it:Acido lattico]] |
|||
[[ja:乳酸]] |
|||
[[kk:Сүт қышқылы]] |
|||
[[ko:젖산]] |
|||
[[lb:Mëllechsaier]] |
|||
[[lt:Pieno rūgštis]] |
|||
[[lv:Pienskābe]] |
|||
[[nl:Melkzuur]] |
|||
[[nn:Mjølkesyre]] |
|||
[[no:Melkesyre]] |
|||
[[pl:Kwas mlekowy]] |
|||
[[pt:Ácido láctico]] |
|||
[[ro:Acid lactic]] |
|||
[[ru:Молочная кислота]] |
|||
[[sh:Mliječna kiselina]] |
|||
[[simple:Lactic acid]] |
|||
[[sk:Kyselina mliečna]] |
|||
[[sl:Mlečna kislina]] |
|||
[[sq:Acidi Laktik]] |
|||
[[sr:Млијечна киселина]] |
|||
[[su:Asam laktat]] |
|||
[[sv:Mjölksyra]] |
|||
[[ta:லாக்டிக் அமிலம்]] |
|||
[[te:లాక్టికామ్లం]] |
|||
[[tr:Laktik asit]] |
|||
[[uk:Молочна кислота]] |
|||
[[vi:Axit lactic]] |
|||
[[zh:乳酸]] |
|||
Revisi terkini sejak 15 April 2024 23.25
| |||
| Nama | |||
|---|---|---|---|
| Nama IUPAC (preferensi)
2-Hydroxypropanoic acid[1] | |||
| Nama lain
Lactic acid[1]
Milk acid | |||
| Penanda | |||
| |||
Model 3D (JSmol)
|
|||
| 3DMet | {{{3DMet}}} | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| Nomor EC | |||
| Nomor RTECS | {{{value}}} | ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Sifat | |||
| C3H6O3 | |||
| Massa molar | 90,08 g·mol−1 | ||
| Titik lebur | 53 °C | ||
| Titik didih | 122 °C (252 °F; 395 K) @ 15 mmHg | ||
| Keasaman (pKa) | 3.86,[2] 15.1[3] | ||
| Termokimia | |||
| Entalpi pembakaran standar ΔcH |
1361.9 kJ/mol, 325.5 kcal/mol, 15.1 kJ/g, 3.61 kcal/g | ||
| Farmakologi | |||
| Kode ATC | G01 QP53AG02 | ||
| Bahaya | |||
| Piktogram GHS |  [4] [4]
| ||
| H315, H318[4] | |||
| P280, P305+351+338[4] | |||
| Senyawa terkait | |||
Anion lain
|
laktat | ||
Kecuali dinyatakan lain, data di atas berlaku pada suhu dan tekanan standar (25 °C [77 °F], 100 kPa). | |||
| Referensi | |||
Asam lakta atau asam susu (CH3-CHOH-COOH) adalah senyawa kimia penting dalam beberapa proses biokimia. Seorang ahli kimia Swedia, Carl Wilhelm Scheele, kali mengisolasinya pada tahun 1780. Secara struktur, ia adalah asam karboksilat dengan satu gugus hidroksil yang menempel pada gugus karboksil. Dalam air, ia terlarut dengan melepas proton (H+), membentuk ion laktat. Asam ini juga larut dalam alkohol dan bersifat menyerap air (higroskopik).
Asam ini memiliki simetri cermin (kiralitas), dengan dua isomer: asam L-(+)-laktat atau asam (S)-laktat dan, cerminannya, iasam D-(-)-laktat atau asam (R)-laktat. Hanya isomer yang pertama (S) aktif secara biologi
Pranala luar
[sunting | sunting sumber]- Corn Plastic to the Rescue Diarsipkan 2013-11-21 di Wayback Machine.
- Lactic Acid: Information and Resources
- Lactic Acid Is Not Muscles' Foe, It's Fuel
- Fitzgerald M (January 26, 2010). "The Lactic Acid Myths". Competitor Running. Diarsipkan dari versi asli tanggal 2018-09-24. Diakses tanggal 2018-09-10.
- ^ a b Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. hlm. 748. doi:10.1039/9781849733069-00648. ISBN 978-0-85404-182-4.
- ^ Dawson RM, et al. (1959). Data for Biochemical Research. Oxford: Clarendon Press.
- ^ Silva AM, Kong X, Hider RC (October 2009). "Determination of the pKa value of the hydroxyl group in the alpha-hydroxycarboxylates citrate, malate and lactate by 13C NMR: implications for metal coordination in biological systems". Biometals. 22 (5): 771–8. doi:10.1007/s10534-009-9224-5. PMID 19288211.
- ^ a b c Sigma-Aldrich Co., DL-Lactic acid.