Plumbagin: Perbedaan antara revisi
Tampilan
Konten dihapus Konten ditambahkan
kTidak ada ringkasan suntingan |
k →Referensi: clean up |
||
| (8 revisi perantara oleh 4 pengguna tidak ditampilkan) | |||
| Baris 18: | Baris 18: | ||
}} |
}} |
||
'''Plumbagin''' (5-Hidroksi-2-metil-1,4-naftokuinon) merupakan senyawa [[naftokuinon]] yang diturunkan dari tumbuh-tumbuhan dan memiliki berbagai aktivitas farmakologis. Telah ditemukan bahwa ia memiliki aktivitas [[mikroorganisme|antimikroba]].<ref>Didry, N., L. Dubrevil & M. Pinkas 1994. Activity of anthraquinonic and naphthoquinonic compounds on oral bacteria. ''Die Pharmazie'' '''49'''(9): 681–683.</ref><ref>Paiva, S.R.d., M.R. Figueiredo, T. V. Aragão, M.A.C. Kaplan 2003. {{PDFlink|[http://www.scielo.br/pdf/mioc/v98n7/v98n7a17.pdf Antimicrobial Activity in Vitro of Plumbagin Isolated from ''Plumbago'' Species.]|511 [[Kibibyte|KiB]]<!-- application/pdf, 523331 bytes -->}} ''Mem Inst Oswaldo Cruz'' '''98'''(7): 959–961.</ref> Pada hewan, ia memiliki efek [[malaria|antimalaria]],<ref>Likhitwitayawuid, K., R. Kaewamatawong, N. Ruangrungsi & J. Krungkrai 1998. Antimalarial naphthoquinones from ''Nepenthes thorelii''. ''Planta Medica'' '''64'''(3): 237–241.</ref> [[karsinogen|antikanker]],<ref>Parimala, R. & P. Sachdanandam 1993. Effect of plumbagin on some glucose metabolizing enzymes studied in rats in experimental hepatoma. ''Molecular and Cellular Biochemistry'' '''12'''(1): 59–63.</ref><ref>Hsu, Y.-L., C.-Y. Cho, P.-L. Kuo, Y.-T. Huang & C.-C. Lin 2006. Plumbagin (5-Hydroxy-2-methyl-1,4-naphthoquinone) Induces Apoptosis and Cell Cycle Arrest in A549 Cells through p53 Accumulation via c-Jun NH2-Terminal Kinase-Mediated Phosphorylation at Serine 15 in Vitro and in Vivo. ''Journal of Pharmacology and Experimental Therapeutics'' '''318'''(2): 484–494. {{DOI|10.1124/jpet.105.098863}}</ref> |
'''Plumbagin''' (5-Hidroksi-2-metil-1,4-naftokuinon) merupakan senyawa [[naftokuinon]] yang diturunkan dari tumbuh-tumbuhan dan memiliki berbagai aktivitas farmakologis. Telah ditemukan bahwa ia memiliki aktivitas [[mikroorganisme|antimikroba]].<ref>Didry, N., L. Dubrevil & M. Pinkas 1994. Activity of anthraquinonic and naphthoquinonic compounds on oral bacteria. ''Die Pharmazie'' '''49'''(9): 681–683.</ref><ref>Paiva, S.R.d., M.R. Figueiredo, T. V. Aragão, M.A.C. Kaplan 2003. {{PDFlink|[http://www.scielo.br/pdf/mioc/v98n7/v98n7a17.pdf Antimicrobial Activity in Vitro of Plumbagin Isolated from ''Plumbago'' Species.]|511 [[Kibibyte|KiB]]<!-- application/pdf, 523331 bytes -->}} ''Mem Inst Oswaldo Cruz'' '''98'''(7): 959–961.</ref> Pada hewan, ia memiliki efek [[malaria|antimalaria]],<ref>Likhitwitayawuid, K., R. Kaewamatawong, N. Ruangrungsi & J. Krungkrai 1998. Antimalarial naphthoquinones from ''Nepenthes thorelii''. ''Planta Medica'' '''64'''(3): 237–241.</ref> [[karsinogen|antikanker]],<ref>Parimala, R. & P. Sachdanandam 1993. Effect of plumbagin on some glucose metabolizing enzymes studied in rats in experimental hepatoma. ''Molecular and Cellular Biochemistry'' '''12'''(1): 59–63.</ref><ref>Hsu, Y.-L., C.-Y. Cho, P.-L. Kuo, Y.-T. Huang & C.-C. Lin 2006. Plumbagin (5-Hydroxy-2-methyl-1,4-naphthoquinone) Induces Apoptosis and Cell Cycle Arrest in A549 Cells through p53 Accumulation via c-Jun NH2-Terminal Kinase-Mediated Phosphorylation at Serine 15 in Vitro and in Vivo. ''Journal of Pharmacology and Experimental Therapeutics'' '''318'''(2): 484–494. {{DOI|10.1124/jpet.105.098863}}</ref> [[jantung|kardiotonik]],<ref>Itoigawa, M., K. Takeya & H. Furukawa 1991. Cardiotonic action of plumbagin on guinea-pig [[papillary muscle]]. ''Planta Medica'' '''57'''(4): 317–319.</ref> [[fertelitas|anti kesuburan]],<ref>Bhargava, S.K. 1984. Effects of plumbagin on reproductive function of male dog. ''Indian Journal of Experimental Biology'' '''22'''(3): 153–156.</ref> dan [[aterosklerosis|anti-aterosklerosis]].<ref>Ding, Y., Z.-J. Chen, S. Liu, D. Che, M. Vetter, C.-H. Chang 2005. Inhibition of Nox-4 activity by plumbagin, a plant-derived bioactive naphthoquinone. ''Journal of Pharmacy and Pharmacology'' '''57'''(1): 111.</ref> |
||
Nama plumbagin berasal dari genus tumbuhan ''[[Plumbago]]'', yang darinya ia pertama kali diisolasi<ref>Van der Vijver, L.M. 1972. Distribution of plumbagin in the Plumbaginaceae. ''Phytochemistry'' '''11''': 3247–3248.</ref> |
Nama plumbagin berasal dari genus tumbuhan ''[[Plumbago]]'', yang darinya ia pertama kali diisolasi<ref>Van der Vijver, L.M. 1972. Distribution of plumbagin in the Plumbaginaceae. ''Phytochemistry'' '''11''': 3247–3248.</ref> |
||
==Referensi== |
== Referensi == |
||
{{reflist}} |
{{reflist}} |
||
[[ |
[[Kategori:Senyawa aromatik]] |
||
[[ |
[[Kategori:Keton]] |
||
| ⚫ | |||
| ⚫ | |||
[[en:Plumbagin]] |
|||
[[it:Plumbagina]] |
|||
Revisi terkini sejak 16 Desember 2022 00.29
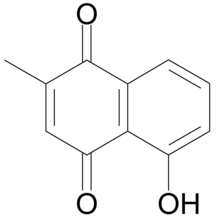
| |
| Nama | |
|---|---|
| Nama IUPAC
5-hidroksi-2-metil-naftalena-1,4-dion
| |
| Penanda | |
Model 3D (JSmol)
|
|
| 3DMet | {{{3DMet}}} |
| Nomor EC | |
| Nomor RTECS | {{{value}}} |
CompTox Dashboard (EPA)
|
|
| |
| Sifat | |
| C11H8O3 | |
| Massa molar | 188,17942 g/mol |
Kecuali dinyatakan lain, data di atas berlaku pada suhu dan tekanan standar (25 °C [77 °F], 100 kPa). | |
| Referensi | |
Plumbagin (5-Hidroksi-2-metil-1,4-naftokuinon) merupakan senyawa naftokuinon yang diturunkan dari tumbuh-tumbuhan dan memiliki berbagai aktivitas farmakologis. Telah ditemukan bahwa ia memiliki aktivitas antimikroba.[1][2] Pada hewan, ia memiliki efek antimalaria,[3] antikanker,[4][5] kardiotonik,[6] anti kesuburan,[7] dan anti-aterosklerosis.[8]
Nama plumbagin berasal dari genus tumbuhan Plumbago, yang darinya ia pertama kali diisolasi[9]
Referensi
[sunting | sunting sumber]- ^ Didry, N., L. Dubrevil & M. Pinkas 1994. Activity of anthraquinonic and naphthoquinonic compounds on oral bacteria. Die Pharmazie 49(9): 681–683.
- ^ Paiva, S.R.d., M.R. Figueiredo, T. V. Aragão, M.A.C. Kaplan 2003. Antimicrobial Activity in Vitro of Plumbagin Isolated from Plumbago Species.PDF (511 KiB) Mem Inst Oswaldo Cruz 98(7): 959–961.
- ^ Likhitwitayawuid, K., R. Kaewamatawong, N. Ruangrungsi & J. Krungkrai 1998. Antimalarial naphthoquinones from Nepenthes thorelii. Planta Medica 64(3): 237–241.
- ^ Parimala, R. & P. Sachdanandam 1993. Effect of plumbagin on some glucose metabolizing enzymes studied in rats in experimental hepatoma. Molecular and Cellular Biochemistry 12(1): 59–63.
- ^ Hsu, Y.-L., C.-Y. Cho, P.-L. Kuo, Y.-T. Huang & C.-C. Lin 2006. Plumbagin (5-Hydroxy-2-methyl-1,4-naphthoquinone) Induces Apoptosis and Cell Cycle Arrest in A549 Cells through p53 Accumulation via c-Jun NH2-Terminal Kinase-Mediated Phosphorylation at Serine 15 in Vitro and in Vivo. Journal of Pharmacology and Experimental Therapeutics 318(2): 484–494. doi:10.1124/jpet.105.098863
- ^ Itoigawa, M., K. Takeya & H. Furukawa 1991. Cardiotonic action of plumbagin on guinea-pig papillary muscle. Planta Medica 57(4): 317–319.
- ^ Bhargava, S.K. 1984. Effects of plumbagin on reproductive function of male dog. Indian Journal of Experimental Biology 22(3): 153–156.
- ^ Ding, Y., Z.-J. Chen, S. Liu, D. Che, M. Vetter, C.-H. Chang 2005. Inhibition of Nox-4 activity by plumbagin, a plant-derived bioactive naphthoquinone. Journal of Pharmacy and Pharmacology 57(1): 111.
- ^ Van der Vijver, L.M. 1972. Distribution of plumbagin in the Plumbaginaceae. Phytochemistry 11: 3247–3248.
