Rosuvastatin: Perbedaan antara revisi
WanaraLima (bicara | kontrib) Artikel baru tentang topik farmasi yang masih memerlukan tambahan dan pengembangan. Artikel ini merupakan terjemahan wikipedia bahasa Inggris masih perlu ditinjau ulang juga disesuaikan dengan padanan bahasa Indonesia |
Mr. Ibrahem (bicara | kontrib) Fix references, Expend infobox mdwiki.toolforge.org. |
||
| Baris 3: | Baris 3: | ||
<!-- Clinical data -->|pregnancy_AU=D|pregnancy_US=X|routes_of_administration=[[Oral administration|By mouth]] ([[Tablet (pharmacy)|tablets]])|Drugs.com={{drugs.com|monograph|rosuvastatin-calcium}}|MedlinePlus=a603033|licence_EU=<!-- EMA uses INN (or special INN_EMA) -->|DailyMedID=Rosuvastatin|licence_US=<!-- FDA may use generic or brand name (generic name preferred) -->|pregnancy_category=|dependency_liability=|legal_AU=S4|legal_CA=<!-- OTC, Rx-only, Schedule I, II, III, IV, V, VI, VII, VIII -->|legal_UK=POM|legal_US=Rx-only|legal_status=Rx-only |
<!-- Clinical data -->|pregnancy_AU=D|pregnancy_US=X|routes_of_administration=[[Oral administration|By mouth]] ([[Tablet (pharmacy)|tablets]])|Drugs.com={{drugs.com|monograph|rosuvastatin-calcium}}|MedlinePlus=a603033|licence_EU=<!-- EMA uses INN (or special INN_EMA) -->|DailyMedID=Rosuvastatin|licence_US=<!-- FDA may use generic or brand name (generic name preferred) -->|pregnancy_category=|dependency_liability=|legal_AU=S4|legal_CA=<!-- OTC, Rx-only, Schedule I, II, III, IV, V, VI, VII, VIII -->|legal_UK=POM|legal_US=Rx-only|legal_status=Rx-only |
||
<!-- Pharmacokinetic data -->|bioavailability=20%<ref name=PK>{{cite journal|last=Aggarwal|first=RK|author2=Showkathali, R|title=Rosuvastatin calcium in acute coronary syndromes|journal=Expert Opinion on Pharmacotherapy|date=June 2013|volume=14|issue=9|pages=1215–1227|doi=10.1517/14656566.2013.789860|pmid=23574635|url=https://semanticscholar.org/paper/a568d46e234e3510d8819d45122a2e8108896ca0|access-date=29 November 2019|archive-date=29 August 2021|archive-url=https://web.archive.org/web/20210829041855/https://www.semanticscholar.org/paper/Rosuvastatin-calcium-in-acute-coronary-syndromes-Aggarwal-Showkathali/a568d46e234e3510d8819d45122a2e8108896ca0|url-status=live}}</ref><ref name=AHFS2018 />|protein_bound=88%<ref name=PK /><ref name=AHFS2018 />|metabolism=[[Liver]]: [[CYP2C9]] (major) and [[CYP2C19]]-mediated; ~10% metabolized<ref name=PK /><ref name=AHFS2018 />|elimination_half-life=19 hours<ref name=PK /><ref name=AHFS2018 />|excretion=[[Feces]] (90%)<ref name=PK /><ref name=AHFS2018 /> |
|||
<!-- Pharmacokinetic data -->|bioavailability=20%<ref name=PK>{{cite journal|last=Aggarwal|first=RK|author2=Showkathali, R|title=Rosuvastatin calcium in acute coronary syndromes|journal=Expert Opinion on Pharmacotherapy|date=June 2013|volume=14|issue=9|pages=1215–1227|doi=10.1517/14656566.2013.789860|pmid=23574635|url=https://semanticscholar.org/paper/a568d46e234e3510d8819d45122a2e8108896ca0|access-date=29 November 2019|archive-date=29 August 2021|archive-url=https://web.archive.org/web/20210829041855/https://www.semanticscholar.org/paper/Rosuvastatin-calcium-in-acute-coronary-syndromes-Aggarwal-Showkathali/a568d46e234e3510d8819d45122a2e8108896ca0|url-status=live}}</ref><ref name=AHFS2018>{{cite web |title=Rosuvastatin Calcium Monograph for Professionals |url=https://www.drugs.com/monograph/rosuvastatin-calcium.html |website=Drugs.com |publisher=[[American Society of Health-System Pharmacists]] (AHFS) |accessdate=24 December 2018 |archive-date=24 December 2018 |archive-url=https://web.archive.org/web/20181224170456/https://www.drugs.com/monograph/rosuvastatin-calcium.html |url-status=live }}</ref>|protein_bound=88%<ref name=PK>{{cite journal|last=Aggarwal|first=RK|author2=Showkathali, R|title=Rosuvastatin calcium in acute coronary syndromes|journal=Expert Opinion on Pharmacotherapy|date=June 2013|volume=14|issue=9|pages=1215–1227|doi=10.1517/14656566.2013.789860|pmid=23574635|url=https://semanticscholar.org/paper/a568d46e234e3510d8819d45122a2e8108896ca0|access-date=29 November 2019|archive-date=29 August 2021|archive-url=https://web.archive.org/web/20210829041855/https://www.semanticscholar.org/paper/Rosuvastatin-calcium-in-acute-coronary-syndromes-Aggarwal-Showkathali/a568d46e234e3510d8819d45122a2e8108896ca0|url-status=live}}</ref><ref name=AHFS2018>{{cite web |title=Rosuvastatin Calcium Monograph for Professionals |url=https://www.drugs.com/monograph/rosuvastatin-calcium.html |website=Drugs.com |publisher=[[American Society of Health-System Pharmacists]] (AHFS) |accessdate=24 December 2018 |archive-date=24 December 2018 |archive-url=https://web.archive.org/web/20181224170456/https://www.drugs.com/monograph/rosuvastatin-calcium.html |url-status=live }}</ref>|metabolism=[[Liver]]: [[CYP2C9]] (major) and [[CYP2C19]]-mediated; ~10% metabolized<ref name=PK>{{cite journal|last=Aggarwal|first=RK|author2=Showkathali, R|title=Rosuvastatin calcium in acute coronary syndromes|journal=Expert Opinion on Pharmacotherapy|date=June 2013|volume=14|issue=9|pages=1215–1227|doi=10.1517/14656566.2013.789860|pmid=23574635|url=https://semanticscholar.org/paper/a568d46e234e3510d8819d45122a2e8108896ca0|access-date=29 November 2019|archive-date=29 August 2021|archive-url=https://web.archive.org/web/20210829041855/https://www.semanticscholar.org/paper/Rosuvastatin-calcium-in-acute-coronary-syndromes-Aggarwal-Showkathali/a568d46e234e3510d8819d45122a2e8108896ca0|url-status=live}}</ref><ref name=AHFS2018>{{cite web |title=Rosuvastatin Calcium Monograph for Professionals |url=https://www.drugs.com/monograph/rosuvastatin-calcium.html |website=Drugs.com |publisher=[[American Society of Health-System Pharmacists]] (AHFS) |accessdate=24 December 2018 |archive-date=24 December 2018 |archive-url=https://web.archive.org/web/20181224170456/https://www.drugs.com/monograph/rosuvastatin-calcium.html |url-status=live }}</ref>|elimination_half-life=19 hours<ref name=PK>{{cite journal|last=Aggarwal|first=RK|author2=Showkathali, R|title=Rosuvastatin calcium in acute coronary syndromes|journal=Expert Opinion on Pharmacotherapy|date=June 2013|volume=14|issue=9|pages=1215–1227|doi=10.1517/14656566.2013.789860|pmid=23574635|url=https://semanticscholar.org/paper/a568d46e234e3510d8819d45122a2e8108896ca0|access-date=29 November 2019|archive-date=29 August 2021|archive-url=https://web.archive.org/web/20210829041855/https://www.semanticscholar.org/paper/Rosuvastatin-calcium-in-acute-coronary-syndromes-Aggarwal-Showkathali/a568d46e234e3510d8819d45122a2e8108896ca0|url-status=live}}</ref><ref name=AHFS2018>{{cite web |title=Rosuvastatin Calcium Monograph for Professionals |url=https://www.drugs.com/monograph/rosuvastatin-calcium.html |website=Drugs.com |publisher=[[American Society of Health-System Pharmacists]] (AHFS) |accessdate=24 December 2018 |archive-date=24 December 2018 |archive-url=https://web.archive.org/web/20181224170456/https://www.drugs.com/monograph/rosuvastatin-calcium.html |url-status=live }}</ref>|excretion=[[Feces]] (90%)<ref name=PK>{{cite journal|last=Aggarwal|first=RK|author2=Showkathali, R|title=Rosuvastatin calcium in acute coronary syndromes|journal=Expert Opinion on Pharmacotherapy|date=June 2013|volume=14|issue=9|pages=1215–1227|doi=10.1517/14656566.2013.789860|pmid=23574635|url=https://semanticscholar.org/paper/a568d46e234e3510d8819d45122a2e8108896ca0|access-date=29 November 2019|archive-date=29 August 2021|archive-url=https://web.archive.org/web/20210829041855/https://www.semanticscholar.org/paper/Rosuvastatin-calcium-in-acute-coronary-syndromes-Aggarwal-Showkathali/a568d46e234e3510d8819d45122a2e8108896ca0|url-status=live}}</ref><ref name=AHFS2018>{{cite web |title=Rosuvastatin Calcium Monograph for Professionals |url=https://www.drugs.com/monograph/rosuvastatin-calcium.html |website=Drugs.com |publisher=[[American Society of Health-System Pharmacists]] (AHFS) |accessdate=24 December 2018 |archive-date=24 December 2018 |archive-url=https://web.archive.org/web/20181224170456/https://www.drugs.com/monograph/rosuvastatin-calcium.html |url-status=live }}</ref> |
|||
<!-- Chemical and physical data -->|chemical_formula=|C=22|H=28|F=1|N=3|O=6|S=1|molecular_weight=481.539|smiles=OC(=O)C[C@H](O)C[C@H](O)\C=C\c1c(C(C)C)nc(N(C)S(=O)(=O)C)nc1c2ccc(F)cc2|StdInChI_Ref={{stdinchicite|changed|chemspider}}|StdInChI=1S/C22H28FN3O6S/c1-13(2)20-18(10-9-16(27)11-17(28)12-19(29)30)21(14-5-7-15(23)8-6-14)25-22(24-20)26(3)33(4,31)32/h5-10,13,16-17,27-28H,11-12H2,1-4H3,(H,29,30)/b10-9+/t16-,17-/m1/s1|StdInChI_comment=|StdInChIKey_Ref={{stdinchicite|changed|chemspider}}|StdInChIKey=BPRHUIZQVSMCRT-VEUZHWNKSA-N|density=|melting_point=|melting_high=|melting_notes=|boiling_point=|boiling_notes=|solubility=|specific_rotation=}} |
<!-- Chemical and physical data -->|chemical_formula=|C=22|H=28|F=1|N=3|O=6|S=1|molecular_weight=481.539|smiles=OC(=O)C[C@H](O)C[C@H](O)\C=C\c1c(C(C)C)nc(N(C)S(=O)(=O)C)nc1c2ccc(F)cc2|StdInChI_Ref={{stdinchicite|changed|chemspider}}|StdInChI=1S/C22H28FN3O6S/c1-13(2)20-18(10-9-16(27)11-17(28)12-19(29)30)21(14-5-7-15(23)8-6-14)25-22(24-20)26(3)33(4,31)32/h5-10,13,16-17,27-28H,11-12H2,1-4H3,(H,29,30)/b10-9+/t16-,17-/m1/s1|StdInChI_comment=|StdInChIKey_Ref={{stdinchicite|changed|chemspider}}|StdInChIKey=BPRHUIZQVSMCRT-VEUZHWNKSA-N|density=|melting_point=|melting_high=|melting_notes=|boiling_point=|boiling_notes=|solubility=|specific_rotation=}} |
||
'''Rosuvastatin''', adalah obat golongan [[statin]], digunakan untuk mencegah [[penyakit kardiovaskular]] pada individu yang memiliki risiko tinggi dan mengatasi [[Dislipidemia|lipid abnormal]] . <ref name= |
'''Rosuvastatin''', adalah obat golongan [[statin]], digunakan untuk mencegah [[penyakit kardiovaskular]] pada individu yang memiliki risiko tinggi dan mengatasi [[Dislipidemia|lipid abnormal]] . <ref name=AHFS2018>{{cite web |title=Rosuvastatin Calcium Monograph for Professionals |url=https://www.drugs.com/monograph/rosuvastatin-calcium.html |website=Drugs.com |publisher=[[American Society of Health-System Pharmacists]] (AHFS) |accessdate=24 December 2018 |archive-date=24 December 2018 |archive-url=https://web.archive.org/web/20181224170456/https://www.drugs.com/monograph/rosuvastatin-calcium.html |url-status=live }}</ref> Pada penggunaan obat ini direkomendasikan bersama dengan perubahan pola makan, berolahraga, dan menurunkan berat badan. <ref name=AHFS2018 /> Penggunaan obat ini diberikan secara oral. <ref name=AHFS2018 /> |
||
Kejadian efek samping obat yang umum terjadi seperti sakit perut, mual, sakit kepala, dan [[Mialgia|nyeri otot]] . <ref name= |
Kejadian efek samping obat yang umum terjadi seperti sakit perut, mual, sakit kepala, dan [[Mialgia|nyeri otot]] . <ref name=AHFS2018 /> Efek samping yang serius pada penggunaan obat ini yakni [[Rabdomiolisis|rhabdomyolysis]], gangguan hepar, dan [[Diabetes melitus|diabetes]] . <ref name=AHFS2018 /> Obat ini tidak direkomendasikan pada ibu hamil karena, membahayakan janin. <ref name=AHFS2018 /> Seperti golongan statin lainnya, rosuvastatin mekanisme kerjanya dengan menghambat HMG-CoA reduktase, [[enzim]] yang terdapat di [[hati]] yang berperan dalam memproduksi [[kolesterol]] . <ref name=AHFS2018 /> |
||
Obat rosuvastatin pertama kali dipatenkan pada tahun 1991, dan disetujui untuk medis di Amerika Serikat pada tahun 2003. <ref name= |
Obat rosuvastatin pertama kali dipatenkan pada tahun 1991, dan disetujui untuk medis di Amerika Serikat pada tahun 2003. <ref name=AHFS2018 /> <ref name="Fis2006">{{Cite book|last=Fischer|first=Janos|last2=Ganellin|first2=C. Robin|date=2006|url=https://books.google.ca/books?id=FjKfqkaKkAAC&pg=PA473|title=Analogue-based Drug Discovery|publisher=John Wiley & Sons|isbn=9783527607495|page=473|access-date=24 December 2018|archive-url=https://web.archive.org/web/20181224220126/https://books.google.ca/books?id=FjKfqkaKkAAC&pg=PA473|archive-date=24 December 2018|url-status=live}}</ref> Obat ini juga tersedia dalam [[obat generik]] . <ref name=AHFS2018 /> |
||
== Referensi == |
== Referensi == |
||
Revisi per 10 Februari 2024 14.10

| |
|---|---|
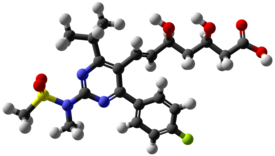
| |
| Nama sistematis (IUPAC) | |
| (3R,5S,6E)-7-[4-(4-Fluorophenyl)-2-(N-methylmethanesulfonamido)-6-(propan-2-yl)pyrimidin-5-yl]-3,5-dihydroxyhept-6-enoic acid | |
| Data klinis | |
| Nama dagang | Crestor, Rosulip, Zuvamor, others |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a603033 |
| Data lisensi | US Daily Med:pranala |
| Kat. kehamilan | D(AU) X(US) |
| Status hukum | Harus dengan resep dokter (S4) (AU) POM (UK) ℞-only (US) ℞ Preskripsi saja |
| Rute | By mouth (tablets) |
| Data farmakokinetik | |
| Bioavailabilitas | 20%[1][2] |
| Ikatan protein | 88%[1][2] |
| Metabolisme | Liver: CYP2C9 (major) and CYP2C19-mediated; ~10% metabolized[1][2] |
| Waktu paruh | 19 hours[1][2] |
| Ekskresi | Feces (90%)[1][2] |
| Pengenal | |
| Kode ATC | ? |
| Data kimia | |
| Rumus | C22H28FN3O6S |
| Massa mol. | 481.539 |
| SMILES | eMolecules & PubChem |
| |
Rosuvastatin, adalah obat golongan statin, digunakan untuk mencegah penyakit kardiovaskular pada individu yang memiliki risiko tinggi dan mengatasi lipid abnormal . [2] Pada penggunaan obat ini direkomendasikan bersama dengan perubahan pola makan, berolahraga, dan menurunkan berat badan. [2] Penggunaan obat ini diberikan secara oral. [2]
Kejadian efek samping obat yang umum terjadi seperti sakit perut, mual, sakit kepala, dan nyeri otot . [2] Efek samping yang serius pada penggunaan obat ini yakni rhabdomyolysis, gangguan hepar, dan diabetes . [2] Obat ini tidak direkomendasikan pada ibu hamil karena, membahayakan janin. [2] Seperti golongan statin lainnya, rosuvastatin mekanisme kerjanya dengan menghambat HMG-CoA reduktase, enzim yang terdapat di hati yang berperan dalam memproduksi kolesterol . [2]
Obat rosuvastatin pertama kali dipatenkan pada tahun 1991, dan disetujui untuk medis di Amerika Serikat pada tahun 2003. [2] [3] Obat ini juga tersedia dalam obat generik . [2]
Referensi
- ^ a b c d e Aggarwal, RK; Showkathali, R (June 2013). "Rosuvastatin calcium in acute coronary syndromes". Expert Opinion on Pharmacotherapy. 14 (9): 1215–1227. doi:10.1517/14656566.2013.789860. PMID 23574635. Diarsipkan dari versi asli tanggal 29 August 2021. Diakses tanggal 29 November 2019.
- ^ a b c d e f g h i j k l m n "Rosuvastatin Calcium Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists (AHFS). Diarsipkan dari versi asli tanggal 24 December 2018. Diakses tanggal 24 December 2018.
- ^ Fischer, Janos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. hlm. 473. ISBN 9783527607495. Diarsipkan dari versi asli tanggal 24 December 2018. Diakses tanggal 24 December 2018.
