Alkaloid vinka: Perbedaan antara revisi
Tidak ada ringkasan suntingan Tag: Suntingan perangkat seluler Suntingan peramban seluler Suntingan seluler lanjutan |
Tidak ada ringkasan suntingan Tag: halaman dengan galat kutipan Suntingan perangkat seluler Suntingan peramban seluler Suntingan seluler lanjutan |
||
| Baris 3: | Baris 3: | ||
==Sumber== |
==Sumber== |
||
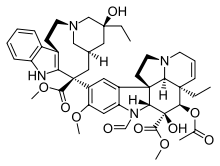
[[Tapak dara]] adalah sumber sejumlah produk alami penting,<ref>{{cite journal|last1 = van der Heijden|first1 = Robert|last2 = Jacobs|first2 = Denise I.|last3 = Snoeijer|first3 = Wim|last4 = Hallard|first4 = Didier|last5 = Verpoorte|first5 = Robert|year = 2004|title = The ''Catharanthus'' alkaloids: Pharmacognosy and biotechnology|journal = [[Current Medicinal Chemistry]]|volume = 11|issue = 5|pages = 607–628|pmid = 15032608|doi = 10.2174/0929867043455846}}</ref> termasuk katarantin dan vindolin<ref>{{cite book|chapter = ''Catharanthus roseus'' L. (Periwinkle): Production of Vindoline and Catharanthine in Multiple Shoot Cultures|first1 = K.|last1 = Hirata|first2 = K.|last2 = Miyamoto|first3 = Y.|last3 = Miura|title = Biotechnology in Agriculture and Forestry 26|series = Medicinal and Aromatic Plants|volume = VI|editor-first = Y. P. S.|editor-last = Bajaj|publisher = [[Springer-Verlag]]|year = 1994|pages = [https://archive.org/details/medicinalaromati0006unse/page/46 46–55]|chapter-url = https://books.google.com/books?id=e64hCDBddowC&pg=PA47|isbn = 9783540563914|url = https://archive.org/details/medicinalaromati0006unse/page/46}}</ref> dan alkaloid vinka yang dihasilkannya: leurosin dan agen kemoterapi [[vinblastin]]<ref>{{cite journal|first1 = Justin E.|last1 = Sears|first2 = Dale L.|last2 = Boger|authorlink2 = Dale L. Boger|title = Total Synthesis of Vinblastine, Related Natural Products, and Key Analogues and Development of Inspired Methodology Suitable for the Systematic Study of Their Structure-Function Properties|journal = [[Accounts of Chemical Research]]|year = 2015|volume = 48|issue = 3|pages = 653–662|doi = 10.1021/ar500400w|pmid = 25586069|pmc = 4363169}}</ref> dan [[vinkristin]],<ref>{{cite journal|last1 = Kuboyama|first1 = Takeshi|last2 = Yokoshima|first2 = Satoshi|last3 = Tokuyama|first3 = Hidetoshi|last4 = Fukuyama|first4 = Tohru|title = Stereocontrolled total synthesis of (+)-vincristine|journal = [[Proceedings of the National Academy of Sciences of the United States of America]]|year = 2004|volume = 101|issue = 33|pages = 11966–11970|doi = 10.1073/pnas.0401323101|pmid = 15141084|bibcode = 2004PNAS..10111966K|pmc = 514417|doi-access = free}}</ref> yang kesemuanya dapat diperoleh dari tumbuhan.<ref name = TopicsCurrentChem>{{cite book|title = Metal Catalyzed Reductive C—C Bond Formation: A Departure from Preformed Organometallic Reagents|volume = 279|series = Topics in Current Chemistry|pages = 25–52|year = 2007|chapter = Reductive C—C bond formation after epoxide opening via electron transfer|first1 = Andreas|last1 = Gansäuer|first2 = José|last2 = Justicia|first3 = Chun-An|last3 = Fan|first4 = Dennis|last4 = Worgull|first5 = Frederik|last5 = Piestert|doi = 10.1007/128_2007_130|chapter-url = https://books.google.com/books?id=A5xcVmT9iIQC&pg=PA25|editor-first = Michael J.|editor-last = Krische|editor1-link=Michael J. Krische|publisher = [[Springer Science & Business Media]]|isbn = 9783540728795}}</ref><ref>{{cite book|chapter = Africa's gift to the world|pages = 46–51|chapter-url = https://books.google.com/books?id=aXGmCwAAQBAJ&pg=PA46|title = Botanical Miracles: Chemistry of Plants That Changed the World|first1 = Raymond|last1 = Cooper|first2 = Jeffrey John|last2 = Deakin|publisher = [[CRC Press]]|year = 2016|isbn = 9781498704304}}</ref><ref name = MoleculesReview>{{cite journal|journal = [[Molecules (journal)|Molecules]]|year = 2012|volume = 17|issue = 5|pages = 5893–5914|doi = 10.3390/molecules17055893|title = Modifications on the basic skeletons of vinblastine and vincristine|first1 = Péter|last1 = Keglevich|first2 = Laszlo|last2 = Hazai|first3 = György|last3 = Kalaus|first4 = Csaba|last4 = Szántay|pmid = 22609781|pmc = 6268133|doi-access = free}}</ref><ref>{{cite book|last = Raviña|first = Enrique|title = The evolution of drug discovery: From traditional medicines to modern drugs|year = 2011|publisher = [[John Wiley & Sons]]|isbn = 9783527326693|pages = 157–159|chapter = ''Vinca'' alkaloids|chapter-url = https://books.google.com/books?id=iDNy0XxGqT8C&pg=PA157}}</ref> Agen kemoterapi semi-sintetik yang lebih baru, vinorelbin, digunakan dalam pengobatan kanker paru-paru non-sel kecil dan tidak diketahui terjadi secara alami. Namun, ia dapat dibuat dari vindolin dan katarantin[ |
[[Tapak dara]] adalah sumber sejumlah produk alami penting,<ref>{{cite journal|last1 = van der Heijden|first1 = Robert|last2 = Jacobs|first2 = Denise I.|last3 = Snoeijer|first3 = Wim|last4 = Hallard|first4 = Didier|last5 = Verpoorte|first5 = Robert|year = 2004|title = The ''Catharanthus'' alkaloids: Pharmacognosy and biotechnology|journal = [[Current Medicinal Chemistry]]|volume = 11|issue = 5|pages = 607–628|pmid = 15032608|doi = 10.2174/0929867043455846}}</ref> termasuk katarantin dan vindolin<ref>{{cite book|chapter = ''Catharanthus roseus'' L. (Periwinkle): Production of Vindoline and Catharanthine in Multiple Shoot Cultures|first1 = K.|last1 = Hirata|first2 = K.|last2 = Miyamoto|first3 = Y.|last3 = Miura|title = Biotechnology in Agriculture and Forestry 26|series = Medicinal and Aromatic Plants|volume = VI|editor-first = Y. P. S.|editor-last = Bajaj|publisher = [[Springer-Verlag]]|year = 1994|pages = [https://archive.org/details/medicinalaromati0006unse/page/46 46–55]|chapter-url = https://books.google.com/books?id=e64hCDBddowC&pg=PA47|isbn = 9783540563914|url = https://archive.org/details/medicinalaromati0006unse/page/46}}</ref> dan alkaloid vinka yang dihasilkannya: leurosin dan agen kemoterapi [[vinblastin]]<ref>{{cite journal|first1 = Justin E.|last1 = Sears|first2 = Dale L.|last2 = Boger|authorlink2 = Dale L. Boger|title = Total Synthesis of Vinblastine, Related Natural Products, and Key Analogues and Development of Inspired Methodology Suitable for the Systematic Study of Their Structure-Function Properties|journal = [[Accounts of Chemical Research]]|year = 2015|volume = 48|issue = 3|pages = 653–662|doi = 10.1021/ar500400w|pmid = 25586069|pmc = 4363169}}</ref> dan [[vinkristin]],<ref>{{cite journal|last1 = Kuboyama|first1 = Takeshi|last2 = Yokoshima|first2 = Satoshi|last3 = Tokuyama|first3 = Hidetoshi|last4 = Fukuyama|first4 = Tohru|title = Stereocontrolled total synthesis of (+)-vincristine|journal = [[Proceedings of the National Academy of Sciences of the United States of America]]|year = 2004|volume = 101|issue = 33|pages = 11966–11970|doi = 10.1073/pnas.0401323101|pmid = 15141084|bibcode = 2004PNAS..10111966K|pmc = 514417|doi-access = free}}</ref> yang kesemuanya dapat diperoleh dari tumbuhan.<ref name = TopicsCurrentChem>{{cite book|title = Metal Catalyzed Reductive C—C Bond Formation: A Departure from Preformed Organometallic Reagents|volume = 279|series = Topics in Current Chemistry|pages = 25–52|year = 2007|chapter = Reductive C—C bond formation after epoxide opening via electron transfer|first1 = Andreas|last1 = Gansäuer|first2 = José|last2 = Justicia|first3 = Chun-An|last3 = Fan|first4 = Dennis|last4 = Worgull|first5 = Frederik|last5 = Piestert|doi = 10.1007/128_2007_130|chapter-url = https://books.google.com/books?id=A5xcVmT9iIQC&pg=PA25|editor-first = Michael J.|editor-last = Krische|editor1-link=Michael J. Krische|publisher = [[Springer Science & Business Media]]|isbn = 9783540728795}}</ref><ref>{{cite book|chapter = Africa's gift to the world|pages = 46–51|chapter-url = https://books.google.com/books?id=aXGmCwAAQBAJ&pg=PA46|title = Botanical Miracles: Chemistry of Plants That Changed the World|first1 = Raymond|last1 = Cooper|first2 = Jeffrey John|last2 = Deakin|publisher = [[CRC Press]]|year = 2016|isbn = 9781498704304}}</ref><ref name = MoleculesReview>{{cite journal|journal = [[Molecules (journal)|Molecules]]|year = 2012|volume = 17|issue = 5|pages = 5893–5914|doi = 10.3390/molecules17055893|title = Modifications on the basic skeletons of vinblastine and vincristine|first1 = Péter|last1 = Keglevich|first2 = Laszlo|last2 = Hazai|first3 = György|last3 = Kalaus|first4 = Csaba|last4 = Szántay|pmid = 22609781|pmc = 6268133|doi-access = free}}</ref><ref>{{cite book|last = Raviña|first = Enrique|title = The evolution of drug discovery: From traditional medicines to modern drugs|year = 2011|publisher = [[John Wiley & Sons]]|isbn = 9783527326693|pages = 157–159|chapter = ''Vinca'' alkaloids|chapter-url = https://books.google.com/books?id=iDNy0XxGqT8C&pg=PA157}}</ref> Agen kemoterapi semi-sintetik yang lebih baru, vinorelbin, digunakan dalam pengobatan kanker paru-paru non-sel kecil dan tidak diketahui terjadi secara alami. Namun, ia dapat dibuat dari vindolin dan katarantin<ref name = MoleculesReview /><ref>{{cite journal|journal = [[Clinical Medicine Insights: Oncology]]|year = 2011|volume = 5|pages = 131–144|doi = 10.4137/CMO.S5074|pmc = 3117629|title = Safety and efficacy of vinorelbine in the treatment of non-small cell lung cancer|first1 = Bryan A.|last1 = Faller|first2 = Trailokya N.|last2 = Pandi|pmid=21695100}}</ref> atau dari leurosin,<ref name = Anhydro /> dalam kedua kasus tersebut melalui sintesis anhidrovinblastin, yang "dapat dianggap sebagai perantara utama untuk sintesis vinorelbin."<ref name = MoleculesReview /> Jalur leurosin menggunakan pereaksi Nugent – RajanBabu dalam de-oksigenasi leurosin yang sangat kemoselektif. Anhidrovinblastin kemudian direaksikan secara berurutan dengan [[N-bromosuksinimida]] dan [[asam trifluoroasetat]] diikuti dengan perak tetrafluoroborat untuk menghasilkan vinorelbin.<ref name = anhydro2vinorelbine /> |
||
[[File:Vinorelbine from leurosine and from catharanthine plus vindoline.jpg|center|1000px]] |
[[File:Vinorelbine from leurosine and from catharanthine plus vindoline.jpg|center|1000px]] |
||
Revisi per 2 September 2024 10.48
Alkaloid vinka adalah seperangkat agen alkaloid anti-mitosis dan anti-mikrotubulus yang aslinya berasal dari tapak dara dan tumbuhan vinka lainnya. Mereka memblokir polimerisasi beta-tubulin dalam sel yang membelah.
Sumber
Tapak dara adalah sumber sejumlah produk alami penting,[1] termasuk katarantin dan vindolin[2] dan alkaloid vinka yang dihasilkannya: leurosin dan agen kemoterapi vinblastin[3] dan vinkristin,[4] yang kesemuanya dapat diperoleh dari tumbuhan.[5][6][7][8] Agen kemoterapi semi-sintetik yang lebih baru, vinorelbin, digunakan dalam pengobatan kanker paru-paru non-sel kecil dan tidak diketahui terjadi secara alami. Namun, ia dapat dibuat dari vindolin dan katarantin[7][9] atau dari leurosin,[10] dalam kedua kasus tersebut melalui sintesis anhidrovinblastin, yang "dapat dianggap sebagai perantara utama untuk sintesis vinorelbin."[7] Jalur leurosin menggunakan pereaksi Nugent – RajanBabu dalam de-oksigenasi leurosin yang sangat kemoselektif. Anhidrovinblastin kemudian direaksikan secara berurutan dengan N-bromosuksinimida dan asam trifluoroasetat diikuti dengan perak tetrafluoroborat untuk menghasilkan vinorelbin.[11]

Penggunaan
Referensi
- ^ van der Heijden, Robert; Jacobs, Denise I.; Snoeijer, Wim; Hallard, Didier; Verpoorte, Robert (2004). "The Catharanthus alkaloids: Pharmacognosy and biotechnology". Current Medicinal Chemistry. 11 (5): 607–628. doi:10.2174/0929867043455846. PMID 15032608.
- ^ Hirata, K.; Miyamoto, K.; Miura, Y. (1994). "Catharanthus roseus L. (Periwinkle): Production of Vindoline and Catharanthine in Multiple Shoot Cultures". Dalam Bajaj, Y. P. S. Biotechnology in Agriculture and Forestry 26. Medicinal and Aromatic Plants. VI. Springer-Verlag. hlm. 46–55. ISBN 9783540563914.
- ^ Sears, Justin E.; Boger, Dale L. (2015). "Total Synthesis of Vinblastine, Related Natural Products, and Key Analogues and Development of Inspired Methodology Suitable for the Systematic Study of Their Structure-Function Properties". Accounts of Chemical Research. 48 (3): 653–662. doi:10.1021/ar500400w. PMC 4363169
 . PMID 25586069.
. PMID 25586069.
- ^ Kuboyama, Takeshi; Yokoshima, Satoshi; Tokuyama, Hidetoshi; Fukuyama, Tohru (2004). "Stereocontrolled total synthesis of (+)-vincristine". Proceedings of the National Academy of Sciences of the United States of America. 101 (33): 11966–11970. Bibcode:2004PNAS..10111966K. doi:10.1073/pnas.0401323101
 . PMC 514417
. PMC 514417  . PMID 15141084.
. PMID 15141084.
- ^ Gansäuer, Andreas; Justicia, José; Fan, Chun-An; Worgull, Dennis; Piestert, Frederik (2007). "Reductive C—C bond formation after epoxide opening via electron transfer". Dalam Krische, Michael J. Metal Catalyzed Reductive C—C Bond Formation: A Departure from Preformed Organometallic Reagents. Topics in Current Chemistry. 279. Springer Science & Business Media. hlm. 25–52. doi:10.1007/128_2007_130. ISBN 9783540728795.
- ^ Cooper, Raymond; Deakin, Jeffrey John (2016). "Africa's gift to the world". Botanical Miracles: Chemistry of Plants That Changed the World. CRC Press. hlm. 46–51. ISBN 9781498704304.
- ^ a b c Keglevich, Péter; Hazai, Laszlo; Kalaus, György; Szántay, Csaba (2012). "Modifications on the basic skeletons of vinblastine and vincristine". Molecules. 17 (5): 5893–5914. doi:10.3390/molecules17055893
 . PMC 6268133
. PMC 6268133  . PMID 22609781.
. PMID 22609781.
- ^ Raviña, Enrique (2011). "Vinca alkaloids". The evolution of drug discovery: From traditional medicines to modern drugs. John Wiley & Sons. hlm. 157–159. ISBN 9783527326693.
- ^ Faller, Bryan A.; Pandi, Trailokya N. (2011). "Safety and efficacy of vinorelbine in the treatment of non-small cell lung cancer". Clinical Medicine Insights: Oncology. 5: 131–144. doi:10.4137/CMO.S5074. PMC 3117629
 . PMID 21695100.
. PMID 21695100.
- ^ Kesalahan pengutipan: Tag
<ref>tidak sah; tidak ditemukan teks untuk ref bernamaAnhydro - ^ Kesalahan pengutipan: Tag
<ref>tidak sah; tidak ditemukan teks untuk ref bernamaanhydro2vinorelbine
