Hidrogen sianida: Perbedaan antara revisi
Tampilan
Konten dihapus Konten ditambahkan
new page |
Add 1 book for Wikipedia:Pemastian (20240409)) #IABot (v2.0.9.5) (GreenC bot |
||
| (13 revisi perantara oleh 12 pengguna tidak ditampilkan) | |||
| Baris 5: | Baris 5: | ||
| ImageFile = Hydrogen-cyanide-2D.svg |
| ImageFile = Hydrogen-cyanide-2D.svg |
||
| ImageFile_Ref = {{chemboximage|correct|??}} |
| ImageFile_Ref = {{chemboximage|correct|??}} |
||
| ImageSize = |
| ImageSize = 200px |
||
| ImageName = Skeletal formula of hydrogen cyanide with the explicit hydrogen added |
| ImageName = Skeletal formula of hydrogen cyanide with the explicit hydrogen added |
||
| ImageFileL1 = Hydrogen-cyanide-3D-balls.png |
| ImageFileL1 = Hydrogen-cyanide-3D-balls.png |
||
| Baris 55: | Baris 55: | ||
| SolubleOther = Miscible |
| SolubleOther = Miscible |
||
| HenryConstant = 75 μmol Pa<sup>−1</sup> kg<sup>−1</sup> |
| HenryConstant = 75 μmol Pa<sup>−1</sup> kg<sup>−1</sup> |
||
| pKa = 9.21<ref>{{ cite book | author = Perrin, D. D. | title = Ionisation Constants of Inorganic Acids and Bases in Aqueous Solution | edition = 2nd | publisher = Pergamon Press | location = Oxford | year = 1982 }}</ref> |
| pKa = 9.21<ref>{{ cite book | author = Perrin, D. D. | title = Ionisation Constants of Inorganic Acids and Bases in Aqueous Solution | url = https://archive.org/details/ionisationconsta0000perr | edition = 2nd | publisher = Pergamon Press | location = Oxford | year = 1982 }}</ref> |
||
| pKb = 4.79 |
| pKb = 4.79 |
||
| RefractIndex = 1.2675 <ref>{{ cite book | author = Patnaik, P. | title = Handbook of Inorganic Chemicals | publisher = McGraw-Hill | year = 2002 | isbn = 0-07-049439-8 }}</ref> |
| RefractIndex = 1.2675 <ref>{{ cite book | author = Patnaik, P. | title = Handbook of Inorganic Chemicals | publisher = McGraw-Hill | year = 2002 | isbn = 0-07-049439-8 }}</ref> |
||
| Baris 68: | Baris 68: | ||
| DeltaHc = -426.5 kJ mol<sup>−1</sup> |
| DeltaHc = -426.5 kJ mol<sup>−1</sup> |
||
| Entropy = 113.01 J K<sup>−1</sup> mol<sup>−1</sup> |
| Entropy = 113.01 J K<sup>−1</sup> mol<sup>−1</sup> |
||
| HeatCapacity = 71.00 kJ K<sup>−1</sup> mol<sup>−1</sup> (at 27 |
| HeatCapacity = 71.00 kJ K<sup>−1</sup> mol<sup>−1</sup> (at 27 °C)<ref>[http://webbook.nist.gov/chemistry/ NIST Chemistry WebBook]</ref> |
||
}} |
}} |
||
| Section5 = {{Chembox Hazards |
| Section5 = {{Chembox Hazards |
||
| Baris 86: | Baris 86: | ||
}} |
}} |
||
}} |
}} |
||
'''Hidrogen sianida (HCN)''' adalah [[senyawa anorganik]]<ref name=Ullmann>{{ Ullmann | author = Gail, E.; Gos, S.; Kulzer, R.; Lorösch, J.; Rubo, A.; Sauer, M. | title = Cyano Compounds, Inorganic | doi = 10.1002/14356007.a08_159.pub2 }}</ref> dengan [[rumus molekul]] HCN. Senyawa ini berbentuk cairan tak berwarna, dan sangat beracun, dengan [[titik didih]] sedikit |
'''Hidrogen sianida (HCN)''' (juga dikenal sebagai '''Asam Sianida''') adalah [[senyawa anorganik]]<ref name=Ullmann>{{ Ullmann | author = Gail, E.; Gos, S.; Kulzer, R.; Lorösch, J.; Rubo, A.; Sauer, M. | title = Cyano Compounds, Inorganic | doi = 10.1002/14356007.a08_159.pub2 }}</ref> dengan [[rumus molekul]] HCN. Senyawa ini berbentuk cairan tak berwarna, dan sangat beracun, dengan [[titik didih]] sedikit di atas [[suhu ruangan]], {{convert|25,6|°C}}.<ref>http://www.wolframalpha.com/input/?i=boiling+point+of+Hydrogen+cyanide</ref> HCN diproduksi dalam skala industri dan sangat bernilai karena digunakan sebagai bahan baku banyak senyawa kimia mulai [[polimer]] sampai obat-obatan. |
||
==Referensi== |
== Referensi == |
||
{{Reflist|30em}} |
{{Reflist|30em}} |
||
==Pranala luar== |
== Pranala luar == |
||
*Institut national de recherche et de sécurité (1997). "[http://www.inrs.fr/inrs-pub/inrs01.nsf/inrs01_ftox_view/860430FE710FCFD7C1256CE8004F67CB/$File/ft4.pdf Cyanure d'hydrogène et solutions aqueuses]". ''Fiche toxicologique n° 4'', Paris:INRS, 5pp. (PDF file, ''in French'') |
* Institut national de recherche et de sécurité (1997). "[http://www.inrs.fr/inrs-pub/inrs01.nsf/inrs01_ftox_view/860430FE710FCFD7C1256CE8004F67CB/$File/ft4.pdf Cyanure d'hydrogène et solutions aqueuses] {{Webarchive|url=https://web.archive.org/web/20060220084315/http://www.inrs.fr/inrs-pub/inrs01.nsf/inrs01_ftox_view/860430FE710FCFD7C1256CE8004F67CB/$File/ft4.pdf |date=2006-02-20 }}". ''Fiche toxicologique n° 4'', Paris:INRS, 5pp. (PDF file, ''in French'') |
||
*[http://www.inchem.org/documents/icsc/icsc/eics0492.htm International Chemical Safety Card 0492] |
* [http://www.inchem.org/documents/icsc/icsc/eics0492.htm International Chemical Safety Card 0492] |
||
*[http://www.inchem.org/documents/cicads/cicads/cicad61.htm Hydrogen cyanide and cyanides] ([[CICAD]] 61) |
* [http://www.inchem.org/documents/cicads/cicads/cicad61.htm Hydrogen cyanide and cyanides] ([[CICAD]] 61) |
||
*[http://www.npi.gov.au/database/substance-info/profiles/29.html National Pollutant Inventory: Cyanide compounds fact sheet] |
* [http://www.npi.gov.au/database/substance-info/profiles/29.html National Pollutant Inventory: Cyanide compounds fact sheet] {{Webarchive|url=https://web.archive.org/web/20060517035532/http://www.npi.gov.au/database/substance-info/profiles/29.html |date=2006-05-17 }} |
||
*[http://www.cdc.gov/niosh/npg/npgd0333.html NIOSH Pocket Guide to Chemical Hazards] |
* [http://www.cdc.gov/niosh/npg/npgd0333.html NIOSH Pocket Guide to Chemical Hazards] |
||
*[http://www.hpa.org.uk/infections/topics_az/deliberate_release/chemicals/cyanide.pdf#search=%22%22dicobalt%20edetate%22%22 Department of health review] |
* [http://www.hpa.org.uk/infections/topics_az/deliberate_release/chemicals/cyanide.pdf#search=%22%22dicobalt%20edetate%22%22 Department of health review] {{Webarchive|url=https://web.archive.org/web/20110607130633/http://www.hpa.org.uk/infections/topics_az/deliberate_release/chemicals/cyanide.pdf#search=%22%22dicobalt%20edetate%22%22 |date=2011-06-07 }} |
||
{{Senyawa hidrogen}} |
{{Senyawa hidrogen}} |
||
{{Authority control}} |
|||
{{DEFAULTSORT:Hydrogen Cyanide}} |
{{DEFAULTSORT:Hydrogen Cyanide}} |
||
[[ |
[[Kategori:Senyawa anorganik]] |
||
[[ |
[[Kategori:Sianida]] |
||
[[Category:Senyawa hidrogen]] |
|||
Revisi terkini sejak 10 April 2024 02.47
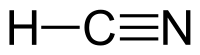
| |||
| |||
| Nama | |||
|---|---|---|---|
| Nama IUPAC | |||
Nama lain
| |||
| Penanda | |||
Model 3D (JSmol)
|
|||
| 3DMet | {{{3DMet}}} | ||
| ChEBI | |||
| ChemSpider | |||
| Nomor EC | |||
| KEGG | |||
| MeSH | Hydrogen+Cyanide | ||
PubChem CID
|
|||
| Nomor RTECS | {{{value}}} | ||
| UNII | |||
| Nomor UN | 1051 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Sifat | |||
| CHN | |||
| Massa molar | 27,03 g·mol−1 | ||
| Penampilan | Cairan transparan atau gas tak berwarna | ||
| Bau | Minyak dari almond pahit | ||
| Densitas | 0.687 g mL−1 | ||
| Bercampur | |||
| Kelarutan dalam etanol | Miscible | ||
| kH | 75 μmol Pa−1 kg−1 | ||
| Keasaman (pKa) | 9.21[3] | ||
| Kebasaan (pKb) | 4.79 | ||
| Indeks bias (nD) | 1.2675 [4] | ||
| Viskositas | 201 μPa s | ||
| Struktur | |||
| Linear | |||
| 2.98 D | |||
| Termokimia | |||
| Kapasitas kalor (C) | 71.00 kJ K−1 mol−1 (at 27 °C)[5] | ||
| Entropi molar standar (S |
113.01 J K−1 mol−1 | ||
| Entalpi pembentukan standar (ΔfH |
109.9 kJ mol−1 | ||
| Entalpi pembakaran standar ΔcH |
-426.5 kJ mol−1 | ||
| Bahaya | |||
Klasifikasi UE (DSD) (usang)
|
|||
| Frasa-R | R12, R26/27/28, R50/53 | ||
| Frasa-S | (S1/2), S16, S36/37, S38, S45, S53, S59, S61 | ||
| Titik nyala | −178 °C (−288,4 °F; 95,1 K) | ||
| Senyawa terkait | |||
Kecuali dinyatakan lain, data di atas berlaku pada suhu dan tekanan standar (25 °C [77 °F], 100 kPa). | |||
| Referensi | |||
Hidrogen sianida (HCN) (juga dikenal sebagai Asam Sianida) adalah senyawa anorganik[6] dengan rumus molekul HCN. Senyawa ini berbentuk cairan tak berwarna, dan sangat beracun, dengan titik didih sedikit di atas suhu ruangan, 25,6 °C (78,1 °F).[7] HCN diproduksi dalam skala industri dan sangat bernilai karena digunakan sebagai bahan baku banyak senyawa kimia mulai polimer sampai obat-obatan.
Referensi[sunting | sunting sumber]
- ^ "Hydrogen Cyanide - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 16 September 2004. Identification. Diakses tanggal 2012-06-04.
- ^ "hydrogen cyanide (CHEBI:18407)". Chemical Entities of Biological Interest. UK: European Bioinformatics Institute. 18 October 2009. Main. Diakses tanggal 2012-06-04.
- ^ Perrin, D. D. (1982). Ionisation Constants of Inorganic Acids and Bases in Aqueous Solution (edisi ke-2nd). Oxford: Pergamon Press.
- ^ Patnaik, P. (2002). Handbook of Inorganic Chemicals. McGraw-Hill. ISBN 0-07-049439-8.
- ^ NIST Chemistry WebBook
- ^ Gail, E.; Gos, S.; Kulzer, R.; Lorösch, J.; Rubo, A.; Sauer, M. (2005), "Cyano Compounds, Inorganic", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a08_159.pub2
- ^ http://www.wolframalpha.com/input/?i=boiling+point+of+Hydrogen+cyanide
Pranala luar[sunting | sunting sumber]
- Institut national de recherche et de sécurité (1997). "Cyanure d'hydrogène et solutions aqueuses Diarsipkan 2006-02-20 di Wayback Machine.". Fiche toxicologique n° 4, Paris:INRS, 5pp. (PDF file, in French)
- International Chemical Safety Card 0492
- Hydrogen cyanide and cyanides (CICAD 61)
- National Pollutant Inventory: Cyanide compounds fact sheet Diarsipkan 2006-05-17 di Wayback Machine.
- NIOSH Pocket Guide to Chemical Hazards
- Department of health review Diarsipkan 2011-06-07 di Wayback Machine.


